Equilibrium Constant in Gaseous Systems
`=>` So far we have expressed equilibrium constant of the reactions in terms of molar concentration of the reactants and products, and used symbol, `color{red}(K_c)` for it.
`=>` For reactions involving gases, however, it is usually more convenient to express the equilibrium constant in terms of partial pressure.
The ideal gas equation is written as,
`color{red}(pV = nRT)`
`=> color{red}(p = n/V RT)`
Here, `color{red}(p)` is the pressure in `color{red}(Pa, n)` is the number of moles of the gas, `color{red}(V)` is the volume in `m^3` and
`color{red}(T)` is the temperature in Kelvin Therefore, `color{red}(n/V)` is concentration expressed in `color{red}(mol//m^3)`
`=>` If concentration `color{red}(c)`, is in `color{red}(mol//L)` or `color{red}(mol//dm^3)`, and `color{red}(p)` is in bar then `color{red}(p = cRT),`
`=>` We can also write `color{red}(p = [gas]RT.)` Here, `color{red}(R= 0.0831)` bar litre/mol K
`=>` At constant temperature, the pressure of the gas is proportional to its concentration i.e., `color{red}(p prop text([gas]))`
`=>` For reaction in equilibrium
`color{red}(H_2 (g) +I_2 (g) ⇌ 2HI (g))`
We can write either `color{red}(K_c = ([ HI (g) ]^2)/([H_2(g) ] [I_2(g)]))`
or `color{red}(K_c = (p_(HI))^2/{(p_(H_2)) (p_(I_2))})` .........(7.12)
Further, since `color{red}(p_(HI) = [HI(g) ] RT)`
`color{red}(p_(I_2) = [I_2(g)]RT)`
`color{red}(p_(H_2) = [H_2(g)]RT)`
Therefore, `color{red}(K_p = (p_(HI))^2/{(p_(H_2))(p_(I_2))} = ([HI(g)]^2 [RT]^2)/{[H_2(g)]RT .[I_2(g)]RT})`
`= color{red}(([HI(g)]^2)/([H_2(g)][I_2(g)]) = K_c)` .......(7.13)
In this example, `color{red}(K_p = K_c)` i.e., both equilibrium constants are equal. However, this is not always the case. For example in reaction
`color{red}(N_2(g) +3H_2(g) ⇌ 2NH_3(g))`
`color{red}(K_p = (p_(NH_3))^2/{(P_(N_2))(p_(H_2))^3})`
` = color{red}(([NH_3(g)]^2 [RT]^2)/([N_2(g)]RT [H_2(g)]^3 (RT)^3})`
` = color{red}({[NH_3(g)]^2 [RT]^(-2)}/([N_2(g)][H_2(g)]^3) = K_c (RT)^(-2))`
or `color{red}(K_p = K_c(RT)^(-2))` ..........(7.14)
`=>` Similarly, for a general reaction
`color{red}(a A + b B ⇌ c C + d D)`
`color{red}(K_p = {(p_C^c)(p_D^d)}/{(p_A^a)(p_B^b)} = {[C]^c [D]^d (RT)^(c+d)}/{[A]^a [B]^b(RT)^(a+b)})`
` = color{red}({[C]^c [D]^d}/{[A]^a [B]^b} (RT)^{(c+d)-(a+b)})`
` = color{red}({ [C]^c[D]^d}/{[A]^a[B]^b} (RT)^(Deltan) = K_c (RT)^(Deltan)) ` .............(7.15)
where `color{red}(Deltan = text{(number of moles of gaseous products) – (number of moles of gaseous reactants)})` in the balanced chemical equation.
`=>` It is necessary that while calculating the value of `color{red}(K_p)`, pressure should be expressed in bar because standard state for pressure is 1 bar.
We know that :
1pascal, `color{red}(Pa=1Nm^(–2))`, and 1bar `= color{red}(10^5 Pa)`
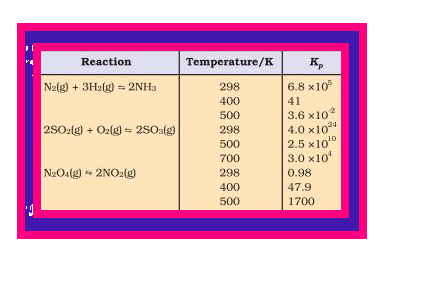
`color{red}(K_p)` values for a few selected reactions at different temperatures are given in Table 7.5
`=>` For reactions involving gases, however, it is usually more convenient to express the equilibrium constant in terms of partial pressure.
The ideal gas equation is written as,
`color{red}(pV = nRT)`
`=> color{red}(p = n/V RT)`
Here, `color{red}(p)` is the pressure in `color{red}(Pa, n)` is the number of moles of the gas, `color{red}(V)` is the volume in `m^3` and
`color{red}(T)` is the temperature in Kelvin Therefore, `color{red}(n/V)` is concentration expressed in `color{red}(mol//m^3)`
`=>` If concentration `color{red}(c)`, is in `color{red}(mol//L)` or `color{red}(mol//dm^3)`, and `color{red}(p)` is in bar then `color{red}(p = cRT),`
`=>` We can also write `color{red}(p = [gas]RT.)` Here, `color{red}(R= 0.0831)` bar litre/mol K
`=>` At constant temperature, the pressure of the gas is proportional to its concentration i.e., `color{red}(p prop text([gas]))`
`=>` For reaction in equilibrium
`color{red}(H_2 (g) +I_2 (g) ⇌ 2HI (g))`
We can write either `color{red}(K_c = ([ HI (g) ]^2)/([H_2(g) ] [I_2(g)]))`
or `color{red}(K_c = (p_(HI))^2/{(p_(H_2)) (p_(I_2))})` .........(7.12)
Further, since `color{red}(p_(HI) = [HI(g) ] RT)`
`color{red}(p_(I_2) = [I_2(g)]RT)`
`color{red}(p_(H_2) = [H_2(g)]RT)`
Therefore, `color{red}(K_p = (p_(HI))^2/{(p_(H_2))(p_(I_2))} = ([HI(g)]^2 [RT]^2)/{[H_2(g)]RT .[I_2(g)]RT})`
`= color{red}(([HI(g)]^2)/([H_2(g)][I_2(g)]) = K_c)` .......(7.13)
In this example, `color{red}(K_p = K_c)` i.e., both equilibrium constants are equal. However, this is not always the case. For example in reaction
`color{red}(N_2(g) +3H_2(g) ⇌ 2NH_3(g))`
`color{red}(K_p = (p_(NH_3))^2/{(P_(N_2))(p_(H_2))^3})`
` = color{red}(([NH_3(g)]^2 [RT]^2)/([N_2(g)]RT [H_2(g)]^3 (RT)^3})`
` = color{red}({[NH_3(g)]^2 [RT]^(-2)}/([N_2(g)][H_2(g)]^3) = K_c (RT)^(-2))`
or `color{red}(K_p = K_c(RT)^(-2))` ..........(7.14)
`=>` Similarly, for a general reaction
`color{red}(a A + b B ⇌ c C + d D)`
`color{red}(K_p = {(p_C^c)(p_D^d)}/{(p_A^a)(p_B^b)} = {[C]^c [D]^d (RT)^(c+d)}/{[A]^a [B]^b(RT)^(a+b)})`
` = color{red}({[C]^c [D]^d}/{[A]^a [B]^b} (RT)^{(c+d)-(a+b)})`
` = color{red}({ [C]^c[D]^d}/{[A]^a[B]^b} (RT)^(Deltan) = K_c (RT)^(Deltan)) ` .............(7.15)
where `color{red}(Deltan = text{(number of moles of gaseous products) – (number of moles of gaseous reactants)})` in the balanced chemical equation.
`=>` It is necessary that while calculating the value of `color{red}(K_p)`, pressure should be expressed in bar because standard state for pressure is 1 bar.
We know that :
1pascal, `color{red}(Pa=1Nm^(–2))`, and 1bar `= color{red}(10^5 Pa)`
`color{red}(K_p)` values for a few selected reactions at different temperatures are given in Table 7.5








